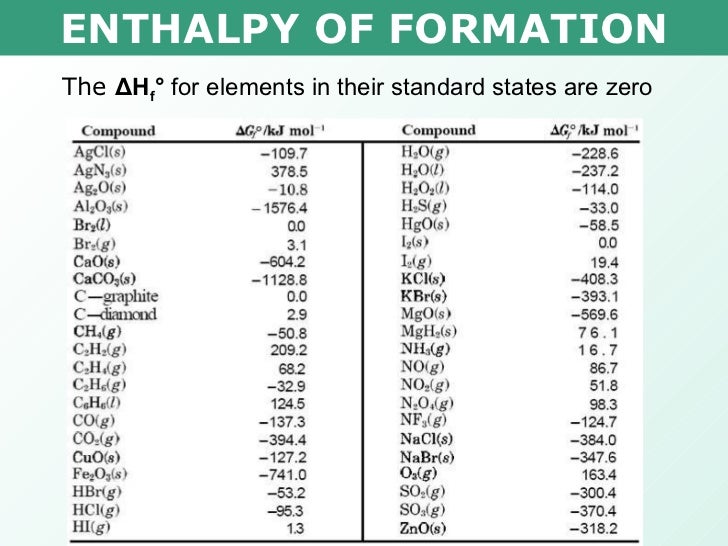
Standard Enthalpy Of Formation In Elements . The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure.
from www.slideshare.net
The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
Tang 03 enthalpy of formation and combustion
Standard Enthalpy Of Formation In Elements A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements.
From www.pinterest.com
Enthalpies of Formation Chemsitry Tutorial Science chemistry Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. A standard enthalpy of. Standard Enthalpy Of Formation In Elements.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation In Elements A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying. Standard Enthalpy Of Formation In Elements.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of. Standard Enthalpy Of Formation In Elements.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID6319219 Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole. Standard Enthalpy Of Formation In Elements.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation In Elements A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation. Standard Enthalpy Of Formation In Elements.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation In Elements A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change. Standard Enthalpy Of Formation In Elements.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpy Of Formation In Elements.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation In Elements A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The. Standard Enthalpy Of Formation In Elements.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of. Standard Enthalpy Of Formation In Elements.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation In Elements A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Enthalpy Of Formation In Elements.
From mavink.com
Enthalpy Table Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies. Standard Enthalpy Of Formation In Elements.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation In Elements The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all. Standard Enthalpy Of Formation In Elements.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation In Elements A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying. Standard Enthalpy Of Formation In Elements.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation. Standard Enthalpy Of Formation In Elements.
From www.chegg.com
Solved Use A Standard Enthalpies Of Formation Table To De... Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of. Standard Enthalpy Of Formation In Elements.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation In Elements The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Standard Enthalpy Of Formation In Elements.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation In Elements The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole. Standard Enthalpy Of Formation In Elements.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation In Elements The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The. Standard Enthalpy Of Formation In Elements.